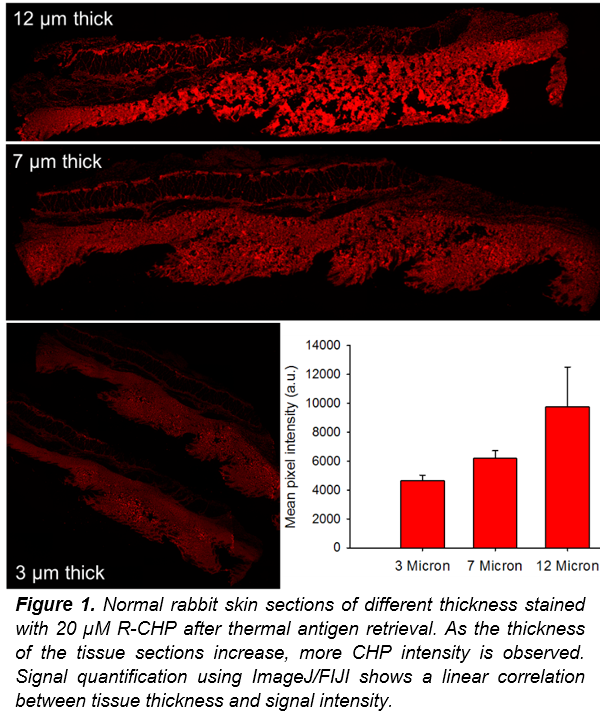
Total Collagen Content:
To use CHPs for evaluating the total collagen content within an FFPE tissue section (after deparaffinization) or a frozen tissue section, the collagen must be fully denatured to allow for CHP binding on all available collagen. We recommend heating the tissue section to thermally denature the collagen. Our tests indicate that the long heating periods used in heat-induced epitope retrieval (HIER) methods are sufficient to completely denature the collagen in the sample (regardless of the buffer used). The tissue sections shown in this application note were placed in 50 mL of citrate buffer in a tissue steamer for 25-45 minutes at 95-100 °C. Alternatively, to denature the collagen content without a steamer, a water bath can be used to heat up a sealed 50 mL tube containing DI water to temperatures over 85 °C. After heating, pipette the hot DI water onto the tissue samples and allow to sit for 5 minutes (repeat 5X).
Image-J/FIJI Macro Steps
View Macro Steps with Comments
Image-J/FIJI Macro Steps
Please follow the steps below to complete the image analysis correctly. The comments describe what each step of the macro code does, and they are shown in green. We have also provided the code without comments so that you can copy and paste it directly into ImageJ by going to Plugins > New > Macro.
NOTE: The user will need to determine an acceptable threshold value to help remove some of the background auto-fluorescence. This macro works best with high signal-to-noise ratio, if there is low signal with lots of background noise or auto-fluorescence, you will need to include additional steps prior to running this macro.
setBatchMode(true); //Tells imageJ that the following code will be run on a batch of files.
output = getDirectory("Output Directory"); //sets the output variable as the user defined directory...
input = getDirectory("Input Directory"); //sets the input variable as the user defined directory...
Dialog.create("File Type");
Dialog.addString("File Suffix: ",".tif"); //tells ImageJ to only use files of type “.tif”...
suffix = Dialog.getString();
processFolder(input); //This is how ImageJ will search through the user specified input folder.
... and so on ...
Copy & Paste Code for ImageJ/FIJI
View Clean Code & Protocol Notes
Copy & Paste Code for ImageJ/FIJI
This is the macro code without comments, ready to be copied directly into ImageJ.
setBatchMode(true);
output = getDirectory("Output Directory");
input = getDirectory("Input Directory");
Dialog.create("File Type");
Dialog.addString("File Suffix: ",".tif");
suffix = Dialog.getString();
processFolder(input);
... and so on ...
This process was designed based of previous literature that focused on the ImageJ/FIJI image quantification tools and methods.4–6 We want to provide the most effective and efficient protocol for our customers. As such, we ask that if you have optimized these steps or found an alternative tool within ImageJ/FIJI that works better, please contact us and we will update this application note and acknowledge your contribution!
Summary
How Does CHP Staining Compare to Other Methods?
CHP staining of fibrotic liver tissue performed just as well if not better than other common stains for detecting collagen in tissue sections. Expert pathologists noted that CHP staining “performed better for collagen detection [than other stains], seemed to have a consistent staining pattern, and was easy to interpret.” In automated image analysis, CHP staining quantified collagen area as accurately as PSR, and more accurately than Masson’s Trichrome, and Herovici’s stain. Overall, CHP is a one-step collagen stain that has high contrast and is easier to read than other collagen histology stains, while maintaining high accuracy.
- Choosing a selection results in a full page refresh.