B-CHP vs. Masson’s Trichrome
vs. Picrosirius Red vs. Herovici’s Stain
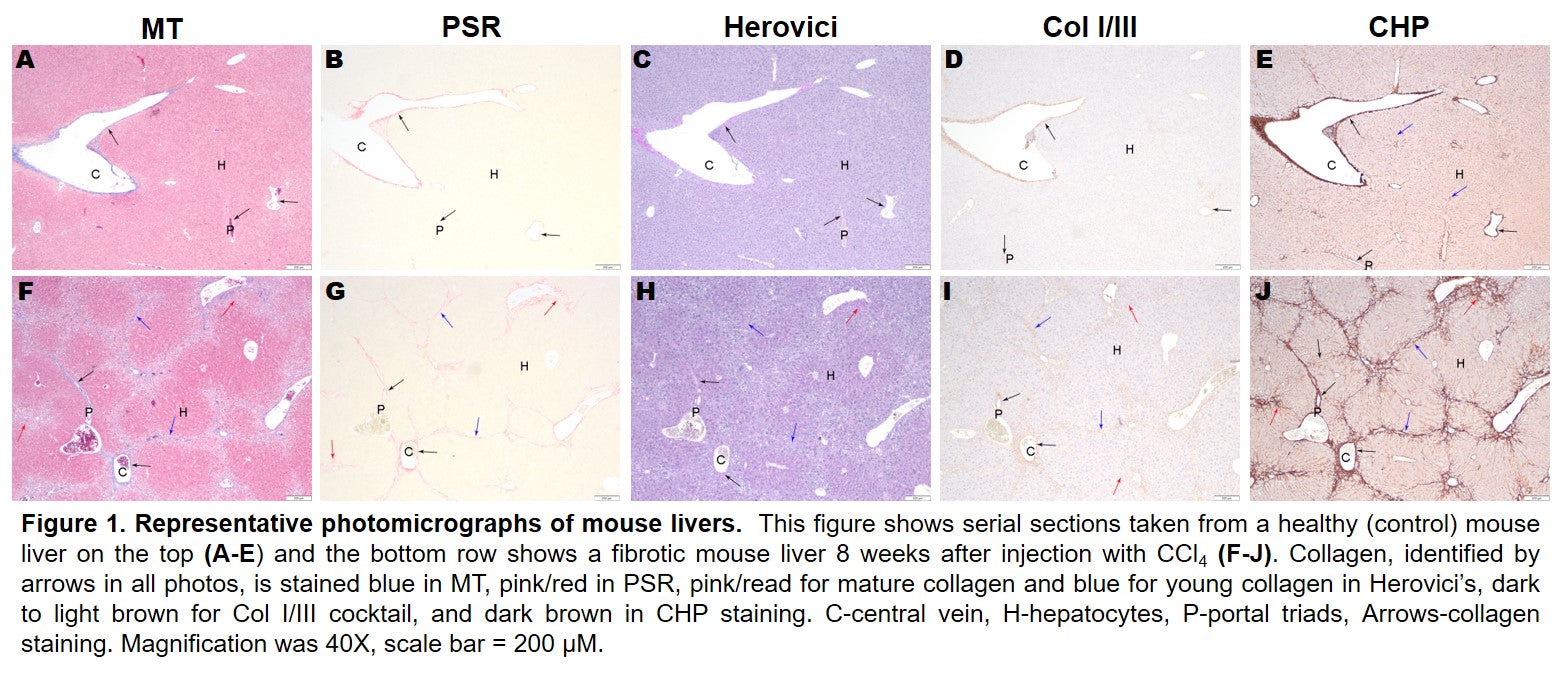
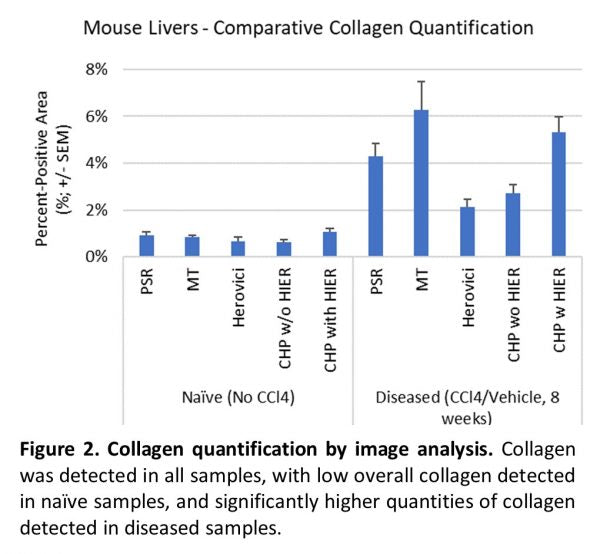
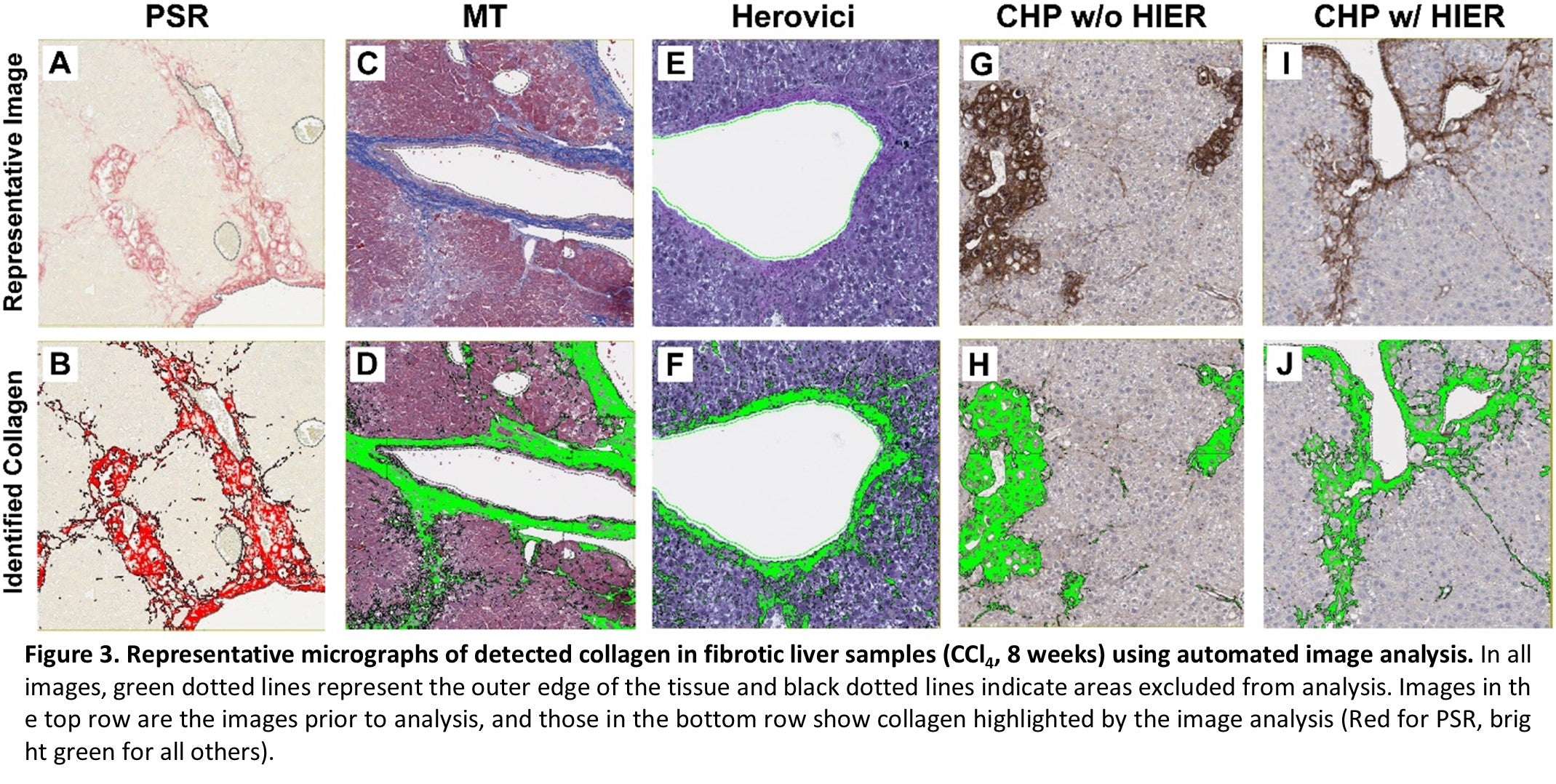
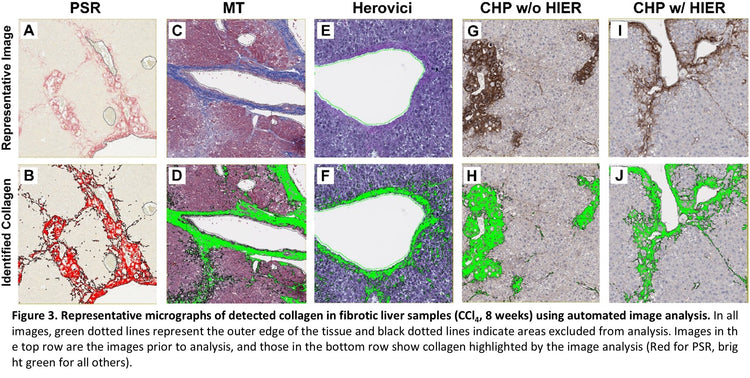
Collagen was detected in all samples and with all stains, but the quantity of collagen varied Figure 2. In naïve samples, the total collagen was low across all samples and staining methods. However, substantial differences were observed between stains in samples from diseased tissue. The most collagen was detected using Masson’s Trichrome. However, as noted above, the weak blue staining of hepatocyte cytoplasm interfered with specific analysis, and the quantity of collagen is likely overrepresented. HistoTox Labs noted: “True staining quantification is likely more similar to PSR detection.” PSR and CHP with HIER were highly similar and likely represent an accurate assessment of total collagen content. Herovici staining was difficult to accurately assess using image analysis and had the smallest area of detected collagen. In addition, consistent with CHP’s specificity for denatured collagen vs all collagen, tissue sections that did not undergo HIER had a lower amount of collagen detected, indicating that both intact and denatured collagen are elevated in the fibrotic tissue. Representative images and analysis from fibrotic samples are shown in Figure 3.
- Choosing a selection results in a full page refresh.