info@3helix.com
Popular Products
F-CHP | Collagen Hybridizing Peptide, 5-FAM Conjugate
- From $218.00 USD
- From $218.00 USD
- Unit price
- / per
R-CHP | Collagen Hybridizing Peptide, Cy3 Conjugate
- From $218.00 USD
- From $218.00 USD
- Unit price
- / per
B-CHP | Collagen Hybridizing Peptide, Biotin Conjugate
- From $218.00 USD
- From $218.00 USD
- Unit price
- / per
Popular Products
F-CHP | Collagen Hybridizing Peptide, 5-FAM Conjugate
- From $218.00 USD
- From $218.00 USD
- Unit price
- / per
R-CHP | Collagen Hybridizing Peptide, Cy3 Conjugate
- From $218.00 USD
- From $218.00 USD
- Unit price
- / per
B-CHP | Collagen Hybridizing Peptide, Biotin Conjugate
- From $218.00 USD
- From $218.00 USD
- Unit price
- / per
Quantifying Collagen Content in Histopathology
by Fluorescent CHP Hybridization
Experimental Protocol
1. FFPE Sections: perform deparaffinization prior to CHP staining by submerging sections for 2 x 5-minute washes in xylenes, 100% ethanol, 95% ethanol, 50% ethanol, and DI water in this order.
2. Perform HIER or purposefully heat denature the tissue for the determination of total collagen content.
3. Create a working solution for CHP staining by dissolving CHP powder in 1× PBS so that the concentration is within a 5-20 μM range. The exact concentration depends on the optimized parameters for the tissue section and the volume needed.
4. Heat CHP solution to 80 °C. Since CHP can self-assemble into homotrimers in solution over time (e.g., during storage at 4 °C) and lose its driving force for collagen hybridization, the trimeric CHP needs to be thermally dissociated to single strands by heating briefly at 80°C.
5. Quickly quench hot CHP solution in an ice water bath (~30 sec) before using it to bind to unfolded collagen in the tissue.
6. Apply quenched CHP solution to tissue section. Completely cover tissue section with CHP staining solution and allow overnight binding at 4 °C in a humidity chamber.
7. Wash sections using 3 x 5-minute washes of 1× PBS or 1× Tween-20.
8. Mount coverslips and image.
For a detailed protocol on tissue preparation please see our User Manual.
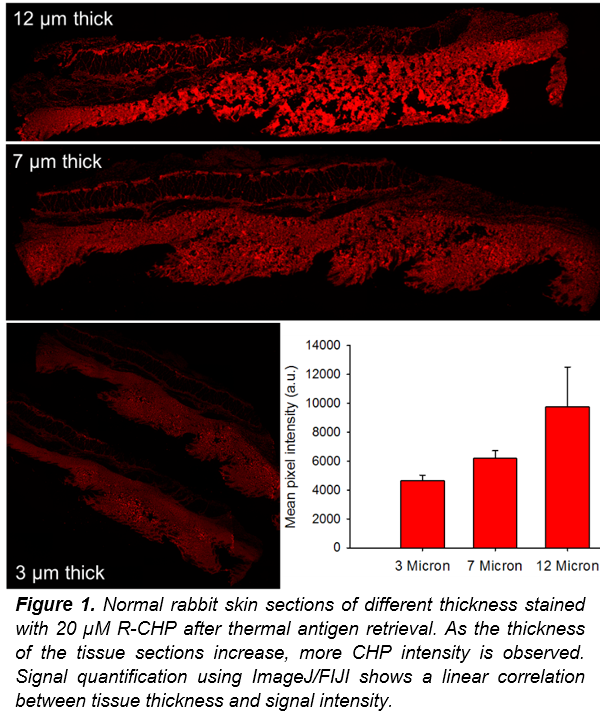
After washing off excess CHP solution, the tissue can be imaged, and imported into ImageJ/FIJI for signal quantification (See below). The signal intensity correlates with the amount of collagen within the sample. As the tissue sections get thicker, they contain more collagen and therefore we expect to see higher signal intensity from CHP binding to denatured collagen strands. In Figure 1, CHP signal is more intense as the tissue gets thicker, confirming that CHP signal correlates to total collagen content. The intensity was determined by measuring the average pixel intensity of the total area imaged after background subtraction. This method will allow researchers to easily visualize and quantify the total collagen content within a tissue section.
- Choosing a selection results in a full page refresh.